Abstract
This study delves into the nutraceutical potential of microalgae in brackishwater aquaculture, with a focus on a case study conducted in the Muttukadu estuary, Tamil Nadu, India. The research involved the collection, identification, culture standardization, nutrient profiling, antioxidant and antibacterial properties of seven microalgal species in the brackishwater ecosystem, including Thalassiosira weissflogii, Chaetoceros gracilis, Nannochloropsis oculata, Chlorella marina, Tetraselmis sp, Isochrysis galbana and Arthrospira maxima. The growth kinetics analysis highlighted N. oculata and C. marina with an extended log phase and significantly higher growth rates. Proximate analysis revealed the highest protein content (62.46 ± 3.45%) in A. maxima. I. galbana showed significantly higher lipid content (33.08 ± 2.18%), while T. weissflogii had 20.11 ± 1.02%. Fatty acid profiling identified the highest percentage of polyunsaturated fatty acids (69.51%) in A. maxima, followed by Tetraselmis sp (56.32%). Methanolic extracts from C. marina and T. weissflogii displayed notably higher total phenolic content. A. maxima exhibited the highest total antioxidant activity, and C-Phycocyanin (CPC) extracts showed the highest inhibition zones against pathogenic bacteria. This study underscored the diversity of commercially important microalgal species in the tropical estuarine brackishwater ecosystem. The nutrient composition analysis emphasized the abundance of essential macronutrients, micronutrients, vitamins, and bioactive compounds. These findings contribute to understanding microalgae's nutraceutical potential in brackishwater aquaculture, suggesting their suitability for large-scale cultivation and utilization in aquaculture and other food-producing industries. Moreover, the bioactive compounds present in these microalgae offer opportunities for developing novel nutraceutical formulations with potential health benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae encompass a varied array of self-sustaining organisms that hold considerable importance within the aquatic food web. They have been used as larval diet in shellfish and finfish hatcheries across the world due to their size (suitable for small-sized larvae), high nutritional composition, high multiplication rate, antioxidant properties and disease resistance. Microalgae synthesize a variety of compounds, mainly biomolecules, which have nutraceutical properties. Nutraceuticals refer to food or food products that provide health and medical benefits, including the prevention and treatment of diseases. These products often contain bioactive compounds such as vitamins, minerals, antioxidants, and other dietary supplements that have potential health benefits beyond basic nutritional value. The bioactive molecules in microalgae have the potential for upscaling at industrial scale. It includes astaxanthin, chlorophyll, phycobiliprotein, beta carotene, lutein and polyunsaturated fatty acids, and they offer significant health and developmental benefits for both humans and animals (Paniagua-Michel 2015). Microalgae, generally inhabit highly competitive and dynamically changing ecosystems. As a result, they synthesize an extensive array of compounds from diverse metabolic pathways. Most of these compounds have been demonstrated to possess various beneficial characteristics, such as antibacterial, antifungal, antiviral, enzyme-inhibiting, immunostimulant, cytotoxic, and anti-plasmodial properties (Ghasemi et al. 2004; Sandeep et al. 2022). For instance, the extracts from Chlorella vulgaris and Chlamydomonas pyrenoidosa exhibit in-vitro antibacterial activities against selected bacteria. Furthermore, in-vitro antibacterial activity has been observed in extracts from green algae, diatoms, and dinoflagellates (Borowitzka and Borowitzka 1988; Sandeep et al. 2023). The ability of Thalassiosira sp to control pathogenic Vibrio sp in shrimp hatcheries has been widely recognized (Sandeep et al. 2021). Quin et al (2013) and Sandeep et al (2021) have demonstrated the capability of this species to inhibit specific pathogenic bacteria. Further, marine microalgae are acknowledged as a valuable, bioactive lipids, particularly those rich in polyunsaturated fatty acids (PUFA), which are effective in preventing and treating various diseases. Notably, n-3 PUFA, such as α-linolenic acid (ALA, C18:3n-3), eicosapentaenoic acid (EPA, C20:5n-3), docosapentaenoic acid (DPA, C22:5n-3), and docosahexaenoic acid (DHA, C22:6n-3), have demonstrated their efficacy in disease prevention and treatment (Abdel-Baky et al. 2002).
Cyanobacteria, one of the most ancient life forms, offer great potential as a class of organisms from which novel bioactive natural compounds can be derived. For example, Spirulina, a well-known nutritional supplement due to its high protein content, contains a primary pigment called phycocyanin (PC). In particular, C-Phycocyanin (Phycocyanin from cyanobacteria, CPC), a water-soluble pigment, is a significant component of spirulina (Boussiba and Richmond 1980). In a recent study, Sandeep et al (2022) demonstrated the positive effects of dietary phycocyanin on the health and growth of Penaeus vannamei, and this finding is particularly relevant in regions where bacterial infections in the shrimps are more prevalent.
The diversity of microalgae in estuarine and coastal marine ecosystems is vast. Applications of microalgae have been extensively studied in different fields (Muller-Feuga 2000; Spolaore et al. 2006; Gupta and Bux 2019; Sandeep et al. 2022). To tap this extensive biosynthetic diversity, researchers have isolated microalgae, and there are several institutions and repositories across the world (Duong et al. 2012). Although almost 50,000 species have been identified and preserved in these collections (Richmond and Qiang 2013), they represent only a fraction of the vast biodiversity of microalgae. To date, only a few microalgal species have been thoroughly studied for their nutraceutical properties and potential applications in aquaculture (Sandeep et al. 2019). To ensure ecological sustainability, it is recommended to utilize native strains for production, as these are better suited to the climatic and environmental conditions of their local habitat. The most suitable local strains for large-scale production are typically those isolated from their natural environments and collected across various seasons, as they are expected to be optimally adapted to specific local conditions. Therefore, a rigorous process of screening and selection is essential to identify the most suitable local strains for efficient microalgae biomass production (Lee et al. 2014). It has been widely acknowledged that native strains exhibit better adaptability to the environmental conditions of their isolation area, particularly when compared to other commercially used species (Abdelaziz et al. 2014; Sandeep et al. 2019).
In this study, the primary objective was to investigate the isolation of promising microalgae species from an estuarine ecosystem. The subsequent steps involve characterizing these isolated microalgae and comprehensively understanding their nutraceutical properties. The significance of this research lies in the scarcity of studies specifically focused on isolating microalgae to evaluate their nutraceutical properties and potential application in marine and brackishwater aquaculture. By doing so, it seeks to contribute foundational information that can be pivotal for the future utilization of various microalgae species to enhance aquaculture production. This research not only addresses the knowledge gap in the isolation and assessment of microalgae with nutraceutical potential but also lays the groundwork for potential advancements in the field of aquaculture through the incorporation of diverse microalgae species.
Materials and methods
Location of the study
Microalgae species isolated in the present study were from the Muttukadu estuarine system in the Chengalpattu District of Tamil Nadu, a Southern state of India (Latitude: 12°48′30.1"N Longitude: 80°14′44.5"E). The Muttukadu estuarine ecosystem is the place where the freshwater Buckingham Canal meets the Bay of Bengal (Fig. 1).
Water quality parameters
Water samples from the different sampling stations were collected for isolation study between 6:30 AM and 8:30 AM. The collected samples were then analyzed following standard laboratory procedures (APHA 2005) to measure various parameters including water temperature (°C), pH, salinity (ppt), dissolved oxygen (mg/L), total ammonia (mg/L), total alkalinity (mg/L), bicarbonate (mg/L) and total Hardness (mg/L).
Isolation and identification
In this study, three methods were employed to isolate various microalgae species, as illustrated in Fig. 2 (Serial dilution, Agar plate streaking, and Micromanipulation). The water samples from the estuarine ecosystem were filtered through a phytoplankton net (10 micron) and the filtered samples underwent serial dilution using specific media such as f/2 media, Walne’s media, and modified Nallayan Research Centre (NRC) media (NaHCO3: 8 g/l; NaCl: 5 g/l; NaNO3: 2.5 g/l; K2SO4: 0.5 g/l; K2HPO4: 0.5 g/l; MgSO47H2O: 0.16 g/l; FeSO47H2O: 0.01 g/l), with various dilutions. The samples were taken at 07:00–08:00 h to ensure the presence of microalgae. After 2–3 weeks, agar streak plating was conducted. The serially diluted test tubes and agar plates were kept in an indoor microalgae laboratory for 1–3 weeks. Periodic microscopic examinations revealed the presence of specific microalgae species. Agar plates were stored in the indoor laboratory at a constant temperature of 24 ± 1 ˚C and illumination of 3,500 ± 100 lx using cool white fluorescent lamps (Philips, 23 W). Light intensity was measured with a lux meter (LX-103, Taiwan), and the photoperiod was fixed at 12:12 h of light and dark periods. If microalgae growth was observed in the dilutions and plates, the cultures were utilized for streaking or re-streaking in agar plates. These plates were again kept in laboratory conditions for 1–2 weeks and observed for colonies. Single colonies were picked using a sterile inoculation loop and re-streaked on freshly prepared agar plates, undergoing 3–4 re-streaking cycles to achieve unialgal growth. For larger species like Arthrospira sp., micromanipulation was employed. A micropipette/pasture pipette was used to separate a single colony, which was then transferred to test tubes with culture media for further multiplication. Microalgae species were identified based on morphological characteristics using taxonomic keys found in standard literatures (Desikachary 1959, 1989; Krishnamurthy 2000). Scanning Electron Microscopy was utilized to examine cellular details of microalgae cells, specifically in the case of Arthrospira maxima, following the method outlined by Kumar et al. (2022) (JSM IT 300LV, JEOL Ltd., Japan).
Pure culture and biomass production
The uncontaminated isolates were cultivated in an indoor microalgae laboratory at a temperature of 24 ± 1 ˚C under 3000–4000 lx illumination. Microalgae culture was conducted using filtered and sterilized seawater. Light intensity was measured using a lux meter (LX-103, Taiwan), and the photoperiod was maintained at 12:12 h Light: Dark. The growth cycles of the isolated species in specific culture media were investigated by daily estimation of cell numbers per ml. Microalgae cell counting was performed using the haemocytometer method (LeGresley and McDermott 2010), with microalgae density expressed as the number of cells ml−1. Sub-culturing of pure cultures occurred at intervals of 7–10 days. Contamination checks were conducted periodically under a microscope. Stock cultures of species (100 ml conical flasks) were without aeration, while aeration was introduced in larger conical flasks (500 ml and above) with filters in the aeration line to prevent contamination.
Nutrient composition
During the logarithmic phase of the growth microalgae was harvested and subjected to freeze-drying. The microalgae's proximate compositions (g/100 g) were determined following the standard procedures outlined by AOAC (2005). The micro-Kjeldahl method (Kjelteck, FOSS tecator, India) was utilized for the estimation of crude protein (CP, expressed in g/100 g), while the assessment of lipid content was conducted through solvent extraction using Soxhlet’s apparatus (SOCS Plus, Pelican, India). To determine the total ash (TA, expressed in g/100 g) content, the microalgae biomasses underwent incineration at 550 °C for 5 h in a muffle furnace. The determination of crude fiber (CF, expressed in g/100 g) content involved acid digestion followed by alkaline fluxing, and the digested sample was subsequently incinerated in a muffle furnace at 550 °C for 5 h. The nitrogen-free extract (NFE, expressed in g/100 g) of the microalgae biomass was calculated using the subtraction method. The following formulas were used for calculating the proximate composition.
(0.1: Normality of acid; 0.014: Molecular mass of N; 6.25: Constant relationship between N and the protein of the sample).
where EE is Ether extract expressed in g/100 g.
The lipid content was estimated by the Bligh and Dyer method (Bligh and Dyer 1959), and the respective fatty acid methyl esters (FAMEs) were prepared and extracted into petroleum ether. Analysis of methyl esters was performed by a gas chromatograph (GC2014 Shimadzu, Japan) on an RTX wax capillary column (100 m length X 0.25 mm I.D X 0.2 µm film thickness). The quantity of fatty acids (mg kg−1) was calculated according to Aziz et al. (2012).
Total phenolic content, total flavonoid content and antioxidant properties
To assess various characteristics of microalgae species, three crucial properties: total phenolic content, total flavonoid content, and total antioxidant activity were measured.
Total phenolic content (TPC)
The quantification of total phenolic content (TPC) in the sample followed the Folin–Ciocalteau (FC) assay as described by Singleton and Rossi (1985), with minor adjustments according to the protocol outlined by Sivaramakrishnan et al (2017). Gallic acid served as the standard, and the phenolic content was expressed as Gallic Acid Equivalents (mgGAE/g) in the macroalgae extract.
Total flavonoid content (TFC)
The assessment of flavonoid content in the microalgae extract followed the spectrophotometric method outlined by Zishen et al (1999), with minor adaptations detailed by Sivaramakrishnan et al (2017). The flavonoid content was quantified as Rutin equivalents in the extract (mg RE/g).
Total antioxidant activity (TAA)
The evaluation of the overall antioxidant activity in methanolic extracts was conducted using the methodology established by Prieto et al (1999), with slight adjustments following the protocol outlined by Sivaramakrishnan et al (2017). The quantification of total antioxidant activity was expressed in milligrams of ascorbic acid per gram of extract.
Antibacterial properties
Bacterial strains, such as Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus, were obtained from the Aquatic Animal Health Division's culture collection at ICAR-CIBA in Chennai, India. The turbidity of the bacterial cultures was adjusted to 0.5 McFarland Standards, equivalent to 107 CFU/ml, and verified by spectrophotometric readings at 600 nm. The antibacterial effectiveness of methanolic extracts from microalgae was assessed against the specified bacteria using the agar well diffusion method outlined by Holder and Boyce (1994). Duplicate wells were separately filled with 50 µl of methanol and DMSO-dissolved microalgae extracts. Negative controls (solvent alone) and positive controls (streptomycin) were also prepared in duplicate. The plates containing bacterial inoculums and extracts were then incubated for 24 h at 37 °C, and the resulting zones of inhibition were measured using a Hi Antibiotic Zone Scale from HiMedia Laboratories.
Preparations of microalgae extract
Algal biomass was harvested at the log phase of the culture by centrifugation and the biomass was freeze-dried immediately. Then the freeze-dried samples were ground in a pestle and mortar using methanol as solvent. The extract was centrifuged at 8000 rpm for 5 min and supernatant was collected and filtered through Whatman filter paper in a vial. Then the pellet was re-centrifuged and the supernatant was collected and filtered again. The collected extract was evaporated by a rotary evaporator. The algal crude extract was stored at -20 °C for further analysis (Sivaramakrishnan et al. 2017; Sandeep et al. 2023).
Statistical methods used
R statistical software (version 4.0.4) was utilized for conducting statistical analyses. The data were presented as mean ± SD. One-way analysis of variance (ANOVA) was carried out, followed by Tukey's HSD post hoc tests, to assess the significance (p < 0.05) of differences in the means of various parameters individually. The generation of all plots was accomplished using the 'ggplot2' package (Wickham 2016) within the R statistical software (ver. 4.0.5).
Results
Water quality parameters
Table 1 shows different water quality parameters from 5 different sampling points of the Muttukadu estuarine ecosystem during the study. The parameters except salinity did not show any significant difference among the sampling points.
Isolation and identification of microalgae
Seven species of microalgae were identified and isolated from the Muttukadu estuarine ecosystem in Tamil Nadu, India. These microalgae are now preserved in the microalgae repository located at the Muttukadu Experimental Station of ICAR-CIBA, as depicted in Fig. 3. The species are Thalassiosira sp, Chaetoceros sp, Tetraselmis sp, Isochrysis sp, Nannochloropsis sp, Chlorella sp, and Arthrospira sp (Fig. 4). Tetraselmis sp, Nannochloropsis sp, and Isochrysis sp were isolated by the agar streak plating method, whereas Thalassiosira sp and Chaetoceros sp were isolated by repeated serial dilution method as they did not form colonies in the agar plates. Arthrospira sp was isolated by using the micromanipulation technique. The cell sizes of Arthrospira sp were observed to be 60–100 microns and single cells were taken with the help of a Pasteur pipette under the microscope. Using morphological characteristics, the microalgae species were identified as Thalassiosira weissflogii, Nannochloropsis oculata, Chlorella marina, Chaetoceros gracilis, Tetraselmis sp, Isochrysis galbana, and Arthrospira maxima (Fig. 4).
Growth kinetics of the selected microalgae isolates
The growth curve of microalgae showed a significantly higher (P < 0.05) growth rate of Nannochloropsis oculata for about 13 days from the start of the culture as compared to other species followed by Chlorella marina (Fig. 5). Both N. oculata and Chlorella marina showed an extended log phase compared to other species. The growth rate of Isochrysis galbana was followed by N. oculata and C. marina. The growth rates of diatoms Thalassiosira weissflogii and Chaetoceros gracilis were comparable to N. oculata and C. marina up to the 7th day. Starting from the 8th day, cultures of T. weissflogii and C. gracilis entered the stationary phase. Notably, the growth rate of T. weissflogii surpassed that of Tetraselmis and C. gracilis. The growth curves illustrated that Tetraselmis sp is a slow-growing microalgae compared to other species, maintaining an extended log phase until the 13th day, albeit with a slower growth rate (Fig. 5).
The growth curve of Arthrospira maxima showed a long lag phase and log phase in the indoor culture conditions (Fig. 6). The growth was observed in a modified NRC medium.
Nutrient composition of microalgae biomass
Proximate composition
A. maxima exhibited the highest protein content at 62.46 ± 3.45%, followed by C. marina (49.48 ± 3.8%), T. weissflogii (43.07 ± 1.78), Tetraselmis sp (42.11 ± 2.55%), N. oculata (38.41 ± 1.85%), I. galbana (34.36 ± 2.86%), and C. gracilis (33.34 ± 2.19%). I. galbana showed significantly higher (P < 0.05) lipid content at 33.08 ± 2.18 compared to others, followed by T. weissflogii (20.11 ± 1.02%), N. oculata (18.35 ± 1.02%), and C. gracilis (14.0 ± 0.97%). The highest nitrogen-free extract (NFE) was observed in C. gracilis (33.35 ± 2.06), followed by Tetraselmis sp (28.43 ± 1.39%), N. oculata and T. weissflogii. No significant difference (P > 0.05) was found in the crude fiber content and total ash content among the various microalgae analyzed (Fig. 7).
Fatty acid profiling
The fatty acid composition analysis of selected microalgae (Table 2) revealed A. maxima had the highest percentage (69.51) of polyunsaturated fatty acids (PUFA), primarily linoleic acid, gamma-linolenic acid (GLA), and dihomo-gamma-linolenic acid. Tetraselmis sp ranked second in PUFA content at 56.32%, dominated by linoleic acid and gamma-linolenic acid (GLA). I. galbana exhibited a noteworthy PUFA percentage (45.29), mainly contributed by alpha-linolenic acid and docosahexaenoic acid (DHA). N. oculata and C. marina displayed PUFA percentages of 37.42 and 29.27, respectively, while C. gracilis exhibited the lowest at 15.17. N. oculata presented the highest percentage of eicosapentaenoic acid (EPA) at 25.2, followed by T. weissflogii. T. weissflogii had the highest monounsaturated fatty acids (MUFA) percentage (39.44), followed by C. gracilis (37.52), N. oculata (30.97), and I. galbana (24.38). Tetraselmis sp had the lowest MUFA percentage at 7.59. C. gracilis exhibited the highest saturated fatty acids (SFA) percentage at 42.38, mainly contributed by palmitic and stearic acids. T. weissflogii had an SFA percentage of 34.56, dominated by palmitic and myristic acids, while N. oculata and C. marina had percentages of 31.61 and 18.86, respectively.
Total phenolic content, total flavonoid content and antioxidant properties
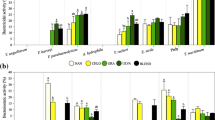
Figure 8 presents diverse total phenolic content, total flavonoid conetnt and antioxidant properties of microalgae extracts. Methanolic extracts of C. gracilis (48.85 ± 3.18) and T. weissflogii (45.83 ± 1.17) demonstrated significantly elevated (P < 0.05) total phenolic content (expressed as mg GAE g−1). Following closely were A. maxima (38.72 ± 2.06), Tetraselmis sp (36.18 ± 1.94), and N. oculata (35.12 ± 1.82). Methanolic extract of A. maxima (8.92 ± 0.92) exhibited significantly higher (P < 0.05) total flavonoid content (expressed as mg RE g−1) compared to other microalgae extracts, followed by Tetraselmis and T. weissflogii. Regarding total antioxidant activity (expressed as mg g−1), A. maxima displayed the highest activity (5.82 ± 0.6), succeeded by T. weissflogii (4.9 ± 0.02) and C. gracilis (4.45 ± 0.28).
Antimicrobial properties
Table 3 outline the antibacterial properties of various microalgae extracts. CPC demonstrated the most substantial zones of inhibition against V. harveyi (20.52 ± 0.35 mm), V. campbellii (18.32 ± 0.52 mm), and V. parahaemolyticus (19.48 ± 0.84), significantly surpassing (P < 0.05) other microalgae extracts. Methanolic extract of T. weissflogii displayed significant inhibitory zones against V. harveyi (15.66 ± 0.18 mm), V. campbellii (14.47 ± 0.78 mm), and V. parahaemolyticus (14.59 ± 0.29 mm), though notably lower (P < 0.05) than CPC. Methanolic extract of Tetraselmis sp exhibited zones of inhibition (13.58 ± 0.26 mm against V. harveyi, 12.40 ± 0.67 mm against V. campbellii, and 12.94 ± 0.73 mm against V. parahaemolyticus) surpassing values for C. gracilis, I. galbana, C. marina, and A. maxima (Table 3). N. oculate showed the lowest inhibition zones against V. harveyi (8.60 ± 0.64 mm), C. marina against V. campbellii (10.50 ± 0.63 mm), and I. galbana against V. parahaemolyticus (12.50 ± 0.56 mm). These findings underscore CPC's significantly superior antibacterial efficacy against the three examined pathogenic bacteria compared to other microalgal extracts.
Discussion
Microalgae, minute photosynthetic organisms, offer a plethora of advantages across diverse industries, including aquaculture (Muller-Feuga 2000; Spolaore et al. 2006; Gupta and Bux 2019; Sandeep et al. 2021). Within the ecosystem, microalgae serve a crucial role as a primary food source for numerous aquatic organisms, contributing significantly to the overall productivity and well-being of aquaculture systems (Hemaiswarya et al. 2011). With an estimated 50,000 or more species of microalgae in existence, only a fraction has been isolated and studied for their chemical composition, with a few hundred receiving attention globally (Duong et al. 2012; Lim et al. 2012). Despite this diversity, the utilization of microalgae species in marine and brackishwater aquaculture remains confined to a limited selection of 10 to 20 species (Muller-Feuga 2000; Sandeep et al. 2019). Microalgae have a rich nutritional profile, containing proteins, lipids, vitamins, and minerals (Hemaiswarya et al. 2011). Different microalgae species may have varying nutritional profiles, and selecting the most suitable ones can enhance the growth, health, and productivity of cultured organisms. Hence the isolation of microalgae strains from various ecosystems has a vital role in aquaculture (Sandeep et al. 2019). Isolating and studying different microalgae species enables the identification of species that have optimal nutritional profiles to meet the dietary requirements of target aquaculture species, thereby promoting balanced and efficient nutrition. Microalgae are often the preferred choice as live feed for larvae due to their small size, high nutritional value, and ability to stimulate natural feeding behaviours in larvae. Isolating and culturing specific microalgae species that are suitable for larval rearing can greatly enhance the survival, growth, and overall success rates of the early life stages of cultured organisms.
Hence the present study was attempted for the isolation of microalgae species from a tropical estuary in southern India. Previous research has focused on the isolation of diverse microalgae from Indian waters, as documented by studies such as those conducted by Dar et al (2019), Sandeep et al (2019), Uma et al (2020) and Raghuraman et al (2021). Several authors have observed that freshly isolated microalgae from a specific region exhibit superior growth performance and biomass production (Duong et al. 2012; Lee et al. 2014; Sandeep et al. 2021). The physicochemical parameters of an ecosystem play a pivotal role in influencing the abundance and growth kinetics of microalgae. Consequently, utilizing local strains of microalgae is advocated for achieving enhanced growth and production. The species isolated in the present study were T. weissflogii, C. gracilis, Tetraselmis sp, I. galbana, N. oculata, C. marina, and A. maxima and earlier studies also reported the presence of similar species in brackishwater ecosystems (Lim et al. 2012; Sandeep et al. 2019; 2022; 2023).
The pure cultures of microalgae are commercially very important for various applications and few culture collections are there in different countries, where the stock cultures are maintained (Duong et al. 2012; Lim et al. 2012). In India, dedicated culture collections of microalgae used in aquaculture are very few (Sandhya et al. 2017; Uma et al. 2020). The present study generated various potential microalgae isolates and they are kept in the microalgal culture repository in pure form for further application.
The growth of the microalgae culture is one of the crucial factors in aquaculture (Brown et al. 1997). The growth rate and ease of culture are significant criteria to be looked upon as a main factor in hatcheries or commercial production units, and all other qualities like nutritional composition are secondary factors for the selection of microalgae. In the present study, the growth of N. oculata, I. galbana, C. marina, T. weissflogii, C. gracilis, Tetraselmis sp, and A. maxima were studied in the indoor culture system (Fig. 5). Earlier studies showed the high growth rates of Chlorella sp and Nannochloropsis sp (Upadhyay et al. 2016). Similarly, the growth rates of N. oculata and C. marina were significantly higher than other microalgae. Among diatoms, T. weissflogii showed a better growth rate (Fig. 5). A similar growth curve of Thalassiosira sp was reported by earlier researchers (Thompson and Harrison 1992; Zhukova 2004). The Tetraselmis sp showed an extended growth curve and slower growth rate than other species. Similar growth kinetics of Tetraselmis sp were reported earlier (D’Souza and Kelly 2000). However, Tetraselmis sp is known for its tolerance toward temperatures and salinities (Raes et al. 2014). To get quality microalgae biomass, it is essential to understand the growth curve of microalgae. The best nutritional composition will be in the log phase, and the nutritional quality reduces considerably when the microalgae reach the stationary phase (Brown et al. 1997). Hence, the harvest time can be determined by understanding the growth curve to get a quality microalgae biomass. The growth curve of A. maxima isolated from the Muttukadu estuary was also estimated (Fig. 6). A similar growth curve was reported earlier in Arthrospira sp (Zhukova 2004; Sandeep et al. 2013; 2019).
Microalgae are rich sources of essential nutrients required for the growth and development of aquatic organisms. They contain high levels of proteins, amino acids, lipids, vitamins, minerals, and pigments that are essential for the health and overall nutritional balance of the organisms being cultured (Spolaore et al. 2006). Proper nutrition is critical for the growth, survival, and overall performance of farmed aquatic organisms. Microalgae provide a natural and highly nutritious food source that can enhance growth rates, improve feed conversion efficiency, and enhance disease resistance in fish, shrimp, and other aquaculture species. The present study evaluated the nutrient profiles of selected microalgae from brackishwater for possible use in brackishwater aquaculture.
Arthrospira sp (spirulina) is a cyanobacterium used as human food for centuries and has been rediscovered recently. Earlier studies revealed the high protein content of spirulina biomass (Hoseini et al. 2013) as 60–70%. The present study revealed the highest percentage of protein in A. maxima (62.46 ± 3.45), followed by C. marina (49.48 ± 3.8). The protein content of Arthrospira sp reported by earlier workers was in a similar range (Sandeep et al. 2013; 2015); however, the protein content in the present study is lower than that reported by Seghiri et al (2019). Earlier researchers (Rani et al. 2018) reported a comparable protein content range of 47–55% in Chlorella sp. In the current study, the protein content in T. weissflogii was determined to be 43.07%. Previous studies (Kiatmetha et al. 2010; Sandeep et al. 2019) have highlighted the superior nutritional quality of T. weissflogii. The protein content in Tetraselmis sp (> 42%) aligns with values reported in prior studies (Abiusi et al. 2014; Kim et al. 2016) and exceeds those documented by D’Souza and Kelly (2000). Nannochloropsis oculata exhibited a protein content of 38.41 ± 1.85% in the present study, consistent with findings from earlier researchers (Hulatt et al. 2017). The protein content of C. gracilis in the present study (> 33%), is similar to the values reported by Pacheco-Vega and Sanchez SaaVedra (2009) in C. muelleri but lower than the values reported by Banerjee et al (2011) in C. calcitrans. In the case of I. galbana, the present study reported a protein content of 34.36 ± 2.86%, aligning with the observations made by Tokuşoglu and Üunal (2003).
The highest lipid content was observed in I. galbana, and these values align with earlier reports (Cañavate et al. 2020). T. weissflogii exhibited a lipid content of 20.11%, consistent with previous findings (Sandeep et al. 2019; 2023). The lipid content of N. oculata in the present study was 18.35 ± 1.02%, and this is higher than the earlier report by Converti et al (2009) and lower than those reported by Huang et al (2013). In the case of C. gracilis, the lipid content in the present study was 14%, surpassing the values reported by Banerjee et al (2011) in C. calcitrans but falling below the levels reported by Pacheco-Vega and Sanchez SaaVedra (2009) in C. muelleri.
The analysis of fatty acid composition in the selected microalgae (Table 2) revealed that Arthrospira maxima exhibited the highest proportion (69.51%) of polyunsaturated fatty acids (PUFA) as a percentage of total fatty acids. This value surpasses those reported by previous researchers (Dibeklioglu et al. 2009), with most studies indicating PUFA percentages in the range of 28.20–55.9%. Earlier investigations suggested the elevated PUFA levels in Arthrospira sp were attributed to increased levels of linoleic acid, gamma-linolenic acid (GLA), and Dihomo-gamma-linolenic acid. The PUFA content of Tetraselmis sp (56.32%) in this study is also higher than that of previous reports (Guzmán et al. 2010; Sandeep et al. 2023). In the case of Isochrysis galbana, the present study observed a PUFA content of 45.29% of total fatty acids, which aligns with values reported by Yoshioka et al (2012). However, the PUFA content in C. gracilis (15.17% of total fatty acids) was lower than that of earlier reports (Bhattacharjya et al. 2020). Fatty acid profiling of T. weissflogii in this study revealed a higher eicosapentaenoic acid (EPA) content (17.77% of total fatty acids), consistent with observations in earlier studies on various Thalassiosira sp species (Thompson and Harrison 1992; Zhukova 2004; Sandeep et al. 2019; 2023). In Tetraselmis sp, the EPA content was 8.39% of total fatty acids, exceeding values reported in some earlier studies (D’Souza and Kelly 2000; Abiusi et al. 2014; Sandeep et al. 2023). The EPA content in C. gracilis in this study is lower than that of earlier reports (Banerjee et al. 2011).
Microalgae are recognized for producing bioactive compounds with potential health advantages, encompassing antioxidants, pigments (such as astaxanthin), fatty acids (including omega-3), and polysaccharides. These compounds can positively impact the health and immune systems of aquaculture species, as highlighted in studies by El-Araby et al (2022) and Sandeep et al (2022). Certain microalgae species generate antimicrobial compounds, antioxidants, and immunostimulants that contribute to disease prevention and enhance the overall health and resilience of cultured organisms. Antioxidants, classified as biological macromolecules, play a role in safeguarding living organisms or their biological components against oxidative radicals. Many photosynthetic microorganisms, including microalgae, have recently been identified as highly reproducible strains producing diverse phenolic compounds and antioxidants. Numerous researchers have elucidated the polyphenolic and antioxidant components of microalgae, as discussed by Choochote et al (2014). Understanding the antioxidant properties is crucial for assessing the potential of a microalgae species as a nutraceutical in aquaculture. Key properties such as total phenolic content, total flavonoid content, and total antioxidant activity are vital parameters for screening potential microalgae for application in aquaculture as nutraceuticals. In the current study, methanolic microalgae extracts were utilized to assess these properties, following methodologies outlined in earlier reports (Hemalatha et al. 2013; Widowati et al. 2017).
In the current investigation, the methanolic extracts of Chlorella marina exhibited a total phenolic content of 48.85 ± 3.18 mg GAE g−1, surpassing values reported in prior research by Hemalatha et al (2013). Similarly, the methanolic extract of T. weissflogii in this study recorded a higher total phenolic content compared to earlier findings by Bhattacharjya et al (2020). However, there are reports of microalgae, such as Scenedesmus rubescens, displaying exceptionally high total phenolic content of up to 48.57 ± 3.99 mg GAE g−1 (Morowvat and Ghasemi 2016). The total phenolic content of Tetraselmis sp in the present study was 36.18 ± 1.9 mg GAE g−1, which exceeded values reported earlier by Maadane et al (2015) and Widowati et al (2017). Meanwhile, the total phenolic content of N. oculata in the present study resembles values reported by Maadane et al (2015) in N. gaditana. Numerous studies have indicated that various cyanobacteria and microalgae possess significant free radical scavenging abilities, potentially linked to the presence of phenolic compounds within them, as highlighted in the work of Jerez-Martel et al (2017).
In the current study, the highest reported total flavonoid content was found in the methanolic extract of A. maxima (8.92 ± 0.92 mg RE g−1), surpassing values reported by Seghiri et al (2019) but falling below those reported by Dianursanti et al. (2020). The total flavonoid content of the methanolic extract of Tetraselmis sp in this study was 7.26 ± 0.05 mg RE g−1, which exceeded earlier reported values (Haoujar et al. 2019). Additionally, the total flavonoid content of the methanolic extract of T. weissflogii in this study was 6.56 ± 0.62 mg RE g−1, higher than the values reported by Bhattacharjya et al (2020). It is worth noting that Haoujar et al (2019) reported higher total flavonoid content in the methanolic extract of N. gaditana than observed in the present study.
Microalgae, aside from their nutritional richness, often exhibit antibacterial properties. In this study, the methanolic extract of A. maxima showed the highest zones of inhibition against all three bacteria tested: V. harveyi (20.52 ± 0.35 mm), V. campbellii (18.32 ± 0.52 mm), and V. parahaemolyticus (19.48 ± 0.84 mm). The results suggests that the antibacterial activity is primarily attributed to the compound CPC, and its potential efficacy against specific pathogens warrants further exploration. The methanolic extract of T. weissflogii also demonstrated a zone of inhibition against V. harveyi (15.66 ± 0.18 mm), V. campbellii (14.47 ± 0.78 mm), and V. parahaemolyticus (14.59 ± 0.29 mm). Similarly, the methanolic extract of Tetraselmis sp exhibited inhibition zones of 13.58 ± 0.26 mm against V. harveyi, 12.40 ± 0.67 mm against V. campbellii, and 12.94 ± 0.73 mm against V. parahaemolyticus, indicating significant antibacterial properties against various pathogenic bacteria, in line with findings from prior research (Guzmán et al. 2019; Bhattacharjya et al. 2020 and Widowati et al. 2021).
Conclusion
Exploring the potential isolates of microalgae from our ecosystem is having great importance as the new isolates will provide better nutrition and health to aquaculture species. The applications of microalgae in brackishwater aquaculture as nutraceuticals have immense potential for the improved production and health of shrimp. Future research should utilize nutraceutical compounds extracted from these potential microalgae (Thalassiosira, Tetraselmis, Chaetoceros, and Arthrospira) as feed additives in larval and nursery diets. This should be extensively evaluated for their efficacy and practical utility through multi-location field trials. Moreover, commercial shrimp hatcheries should evolve strategies by using combinations of microalgae as live feed for improved penaeid shrimp larval nutrition resulting in improved survival in hatcheries.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdelaziz AE, Leite GB, Belhaj MA, Hallenbeck PC (2014) Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Biores Technol 157:140–148. https://doi.org/10.1016/j.biortech.2014.01.114
Abdel-Baky HH, Shallan MA, El-Baroty G, El-Baz FK (2002) Volatile compounds of the microalga Chlorella vulgaris and their phytotoxic effect. Pak J Biol Sci 5:61–65. https://doi.org/10.3923/pjbs.2002.61.65
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, Ottavio MD, Tredici MR (2014) Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F and M-M33 grown with LEDs of different colours. Biotechnol Bioeng 111(5):956–964. https://doi.org/10.1002/bit.25014
AOAC (2005) Official Methods of Analysis of AOAC International, 18th Ed., AOAC International, Gaithersburg, MD, USA
APHA (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association, Washington, DC, USA
Aziz NA, Azlan A, Ismail A, Mohd Alinafiah S, Razman MR (2012) Quantitative determination of fatty acids in marine fish and shellfish from warm water of straits of Malacca for nutraceutical purposes. Biomed Res Int 1–12. https://doi.org/10.1155/2013/284329
Baek SH, Jung SW, Shin K (2011) Effects of temperature and salinity on growth of Thalassiosira pseudonana (Bacillariophyceae) isolated from ballast water. J Freshw Ecol 26(4):547–552. https://doi.org/10.1080/02705060.2011.582696
Bahi A, Ramos-Vega A, Angulo C, Monreal-Escalante E, Guardiola FA (2023) Microalgae with immunomodulatory effects on fish. Rev Aquac. https://doi.org/10.1111/raq.12792
Banerjee S, Hew WE, Khatoon H, Shariff M, Fatimah MdY (2011) Growth and proximate composition of tropical marine Chaetoceros calcitrans and Nannochloropsis oculata cultured outdoors and under laboratory conditions. Afr J Biotech 10(8):1375–1383
Bhattacharjya R, Marella TK, Tiwari A, Saxena A, Singh PK, Mishra B (2020) Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Biores Technol 318:124073. https://doi.org/10.1016/j.biortech.2020.124073
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Borowitzka MA, Borowitzka LJ (1988) Micro-algal biotechnology. Cambridge University Press, New York
Boussiba S, Richmond AE (1980) C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Achieves Microbiol 125(1):143–147. https://doi.org/10.1007/BF00403211
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331. https://doi.org/10.1016/S0044-8486(96)01501-3
Brown MR (2002) Nutritional value and use of microalgae in aquaculture. Avances en Nutrición Acuicola. https://nutricionacuicola.uanl.mx/index.php/acu/article/view/242
Cañavate JP, Hachero-Cruzado I, Pérez-Gavilán C, Fernández-Díaz C (2020) Lipid dynamics and nutritional value of the estuarine strain Isochrysis galbana VLP grown from hypo to hyper salinity. J Appl Phycol 32(6):3749–3766. https://doi.org/10.1007/s10811-020-02258-2
Choochote W, Suklampoo L, Ochaikul D (2014) Evaluation of antioxidant capacities of green microalgae. J Appl Phycol 26(1):43–48. https://doi.org/10.1007/s10811-013-0084-6
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48(6):1146–1151. https://doi.org/10.1016/j.cep.2009.03.006
D’Souza FML, Kelly GJ (2000) Effects of a diet of a nitrogen limited algae (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture 181:311–329. https://doi.org/10.1016/S0044-8486(99)00231-8
Dar RA, Arora M, Phutela UG (2019) Optimization of cultural factors of newly isolated microalga Spirulina subsalsa and its co-digestion with paddy straw for enhanced biogas production. Bioresource Technol Rep 5:185–198. https://doi.org/10.1016/j.biteb.2019.01.009
Desikachary TV (1959) Cyanophyta. Volume 2 of I.C.A.R. monographs on algae. Indian Council of Agricultural Research, New Delhi, pp 686
Desikachary TV (1989) Atlas of diatoms. Madras Sci Found, vol 2. Madras, pp 221
Dianursanti, Nugroho P, Prakasa MB (2020) Comparison of maceration and soxhletation method for flavonoid production from Spirulina platensis as a sunscreen’s raw material. In: AIP Conference Proceedings 2230(1):020006. https://doi.org/10.1063/5.0002806. AIP Publishing LLC
Dibeklioglu H, Koru E, Diraman H (2009) Fatty acid profile of Spirulina platensis used as a food supplement. Israeli J Aquacult-Bamidgeh 61: 20548. http://hdl.handle.net/10524/19280
Duong VT, Li Y, Nowak E, Schenk PM (2012) Microalgae isolation and selection for prospective biodiesel production. Energies 5(6):1835–1849. https://doi.org/10.3390/en5061835
El-Araby DA, Amer SA, Attia GA, Osman A, Fahmy EM, Altohamy DE, Alkafafy M, Elakkad HA, Tolba SA (2022) Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia. Oreochromis Niloticus Aquacult 546:737413. https://doi.org/10.1016/j.aquaculture.2021.737413
Ghasemi Y, Yazdi MT, Shafiee A, Amini M, Shokravi S, Zarrini G (2004) Parsiguine, a novel antimicrobial substance from Fischerella ambigua. Pharm Biol 42(4–5):318–322. https://doi.org/10.1080/13880200490511918
Gupta S, Bux F (2019) Application of microalgae in wastewater treatment. Appl Microalgae Wastewater Treat 1. https://doi.org/10.1007/978-3-030-13909-4
Guzmán F, Wong G, Román T, Cárdenas C, Alvárez C, Schmitt P, Albericio F, Rojas V (2019) Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) Butcher and bactericidal activity improvement. Mar Drugs 17(8):453. https://doi.org/10.3390/md17080453
Guzmán HM, de la Jara VA, Duarte LC, Presmanes KF (2010) Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult Int 18(2):189–199. https://doi.org/10.1007/s10499-008-9235-1
Haoujar I, Cacciola F, Abrini J, Mangraviti D, Giuffrida D, Oulad El Majdoub Y, Kounnoun A, Miceli N, Fernanda Taviano M, Mondello L, Rigano F (2019) The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules 24(22):4037. https://doi.org/10.3390/molecules24224037
Hemaiswarya S, Raja R, Ravi Kumar R, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746. https://doi.org/10.1007/s11274-010-0632-z
Hemalatha A, Girija K, Parthiban C, Saranya C, Anantharaman P (2013) Antioxidant properties and total phenolic content of a marine diatom, Navicula clavata and green microalgae, Chlorella marina and Dunaliella salina. Adv Appl Sci Res 4(5):151–157
Holder IA, Boyce ST (1994) Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 20(5):426–429. https://doi.org/10.1016/0305-4179(94)90035-3
Hoseini SM, Khosravi-Darani K, Mozafari MR (2013) Nutritional and medical applications of spirulina microalgae. Mini Rev Med Chem 13(8):1231–1237. https://doi.org/10.2174/1389557511313080009
Huang X, Huang Z, Wen W, Yan J (2013) Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J Appl Phycol 25(1):129–137. https://doi.org/10.1007/s10811-012-9846-9
Hulatt CJ, Wijffels RH, Bolla S, Kiron V (2017) Production of fatty acids and protein by Nannochloropsis in flat-plate photobioreactors. PLoS One 12(1):e0170440. https://doi.org/10.1371/journal.pone.0170440
Jerez-Martel I, García-Poza S, Rodríguez-Martel G, Rico M, Afonso-Olivares C, Gómez-Pinchetti JL (2017) Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J Food Qual 2017:2924508. https://doi.org/10.1155/2017/2924508
Karlson B, Cusack C, Bresnan E (2010) Microscopic and molecular methods for quantitative phytoplankton analysis. Paris, Intergovernmental Oceanographic Commission of UNESCO, IOC Manuals and Guides 55. https://doi.org/10.25607/OBP-1371
Kiatmetha P, Siangdang W, Bunnag B, Senapin S, Withyachumnarnkul B (2010) Enhancement of survival and metamorphosis rates of Penaeus monodon larvae by feeding with the diatom Thalassiosira weissflogii. Aquacult Int 19:599–609. https://doi.org/10.1007/s10499-010-9375-y
Kim G, Mujtaba G, Lee K (2016) Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 31(3):257–266. https://doi.org/10.4490/algae.2016.31.8.18
Krishnamurthy V (2000) Algae of India and neighbouring countries- Chlorophycota. Oxford and IBH publishing Co. Pvt. Ltd., New Delhi
Kumar TS, Praveena PE, Sivaramakrishnan T, Rajan JJS, Makesh M, Jithendran KP (2022) Effect of Enterocytozoon hepatopenaei (EHP) infection on physiology, metabolism, immunity, and growth of Penaeusvannamei. Aquaculture 553:738105. https://doi.org/10.1016/j.aquaculture.2022.738105
Lee K, Eisterhold ML, Rindi F, Palanisami S, Nam PK (2014) Isolation and screening of microalgae from natural habitats in the midwestern United States of America for biomass and biodiesel sources. J Natural Sci Biol Med 5(2):333. https://doi.org/10.4103/0976-9668.136178
LeGresley M, McDermott G (2010) Counting chamber methods for quantitative phytoplankton analysis: haemocytometer, palmer-maloney cell and sedgewick-rafter cell. In: Karlson B, Cusack C, Bresnan E (eds) Microscopic and molecular methods for quantitative phytoplankton analysis. IOC Manuals and Guides No. 55. UNESCO, Paris, pp 25–30
Lim DK, Garg S, Timmins M, Zhang ES, Thomas-Hall SR, Schuhmann H, Li Y, Schenk PM (2012) Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS One 7(7):e40751. https://doi.org/10.1371/journal.pone.0040751
Maadane A, Merghoub N, Ainane T, El Arroussi H, Benhima R, Amzazi S, Bakri Y, Wahby I (2015) Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J Biotechnol 215:13–19. https://doi.org/10.1016/j.jbiotec.2015.06.400
Morowvat MH, Ghasemi Y (2016) Evaluation of antioxidant properties of some naturally isolated microalgae: Identification and characterization of the most efficient strain. Biocatal Agric Biotechnol 8:263–269. https://doi.org/10.1016/j.bcab.2016.09.010
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12(3–5):527–534. https://doi.org/10.1023/A:1008106304417
Ogbonda KH, Aminigo RE, Abu GO (2007) Influence of aeration and lighting on biomass production and protein biosynthesis in a Spirulina sp. isolated from an oil-polluted brackish water marsh in the Niger Delta, Nigeria. Afr J Biotechnol 6(22):2596–2600. https://doi.org/10.4314/ajb.v6i22.58149
Pacheco-Vega JM, Sanchez SaaVedra MDP (2009) The biochemical composition of Chaetocerosmuelleri (Lemmermann) grown with an agricultural fertilizer. J World Aquacult Soc 40(4):556–560. https://doi.org/10.1111/j.1749-7345.2009.00276.x
Paniagua-Michel J (2015) Microalgal nutraceuticals. In Handbook of marine microalgae (pp. 255–267). Academic Press
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2):337–341. https://doi.org/10.1006/abio.1999.4019
Quin JG, Trent DA, Zhang W, Franco C (2013) Discovery of antimicrobial activities of a marine diatom Thalassiosirarotula. Afr J Microbiol Res 7(50):5687–5696. https://doi.org/10.5897/AJMR12.2183
Raes EJ, Isdepsky A, Muylaert K, Borowitzka MA, Moheimani NR (2014) Comparison of growth of Tetraselmis in a tubular photobioreactor (Biocoil) and a raceway pond. J Appl Phycol 26(1):247–255. https://doi.org/10.1007/s10811-013-0077-5
Raghuraman R, Aiyamperumal B, Anantharaman P (2021) A new record of Spirulina subsalsa (Oersted Ex Gomont, 1892) with molecular profile isolated in Vellar Estuary, Portonovo, South East Coast, Tamil Nadu (India). Acta Ecologica Sinica https://doi.org/10.1016/j.chnaes.2021.04.008
Rakesh S, Dhar DW, Prasanna R, Saxena AK, Saha S, Shukla M, Sharma K (2015) Cell disruption methods for improving lipid extraction efficiency in unicellular microalgae. Eng Life Sci 15(4):443–447. https://doi.org/10.1002/elsc.201400222
Rani K, Sandal N, Sahoo PK (2018) A comprehensive review on Chlorella -its composition, health benefits, market and regulatory scenario. Pharma Innov J 7(7):584–589
Richmond A, Qiang H (2013) Handbook of Microalgal Culture: Applied Phycology and Biotechnology. 2nd ed. Hoboken, N. J., USA: John Wiley & Sons, Ltd; 2013. pp. 719
Safari R, Raftani Amiri Z, Esmaeilzadeh Kenari R (2020) Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran J Fish Sci 19(4): 1911–1927. http://jifro.ir/article-1-2496-fa.html
Sandeep KP, Shukla SP, Vennila A, Purushothaman CS, Manjulekshmi N (2015) Cultivation of Spirulina (Arthrospira) platensis in low cost seawater based medium for extraction of value added pigments. Indian J Geo-Marine Sci 44 (3): 384–393. http://nopr.niscpr.res.in/handle/123456789/34696
Sandeep KP, Avunje S, Dayal JS, Balasubramanian CP, Sawant PB, Chadha NK, Ambasankar K, Vijayan KK (2021) Efficiency of different microalgae as monospecific and bispecific diets in larval rearing of Penaeusindicus with special reference to growth, nutrient composition and antimicrobial activity of microalgae. Aquacult Res 52(11):5146–5154. https://doi.org/10.1111/are.15382. (1–9)
Sandeep KP, Shukla SP, Harikrishna V, Muralidhar AP, Vennila A, Purushothaman CS, Ratheesh Kumar R (2013) Utilization of inland saline water for Spirulina cultivation. J Water Reuse Desalination IWA Publ 3(4):346–356. https://doi.org/10.2166/wrd.2013.102
Sandeep KP, Angel JRJ, Sivaramakrishnan T, Sudhin S, Suganya N, Ananda Raja R, Kumar S, Tomy S, Dayal SJ, Balasubramanian CP, Sawant PB, Shekhar MS, Chadha NK, Ambasankar K (2022) Effect of dietary C-Phycocyanin on growth, survival, haematology, immune response, gut microbiome and disease resistance of Pacific white shrimp, Penaeusvannamei. Aquacult Res 53(17):6292–6309. https://doi.org/10.1111/are.16102
Sandeep KP, Kumaraguru Vasagam KP, Kumararaja P, Syama Dayal J, Sreekanth GB, Ambasankar K, Vijayan KK (2019) Microalgal diversity of a tropical estuary in south India with special reference to isolation of potential species for aquaculture. J Coast Conserv 23(1):253–267. https://doi.org/10.1007/s11852-018-0655-4
Sandeep KP, Sivaramakrishnan T, Sudhin S, Raymond JAJ, Sudheer NS, Ananda Raja R, Sujeet Kumar, Syama Dayal J, Balasubramanian CP, Paramita Banerjee Sawant, Chadha NK, Ambasankar K (2023) Influence of dietary microalgal concentrates on growth, survival and health status of Penaeus vannamei. Aquaculture International accepted on 6th April, 2023. https://doi.org/10.1007/s10499-023-01114-7
Sandhya SV, Preetha K, Nair AV, Antony ML, Vijayan KK (2017) Isolation, characterisation and phylogenetic diversity of culturable bacteria associated with marine microalgae from saline habitats of south India. Aquat Microb Ecol 79(1):21–30. https://doi.org/10.3354/ame01815
Seghiri R, Kharbach M, Essamri A (2019) Functional composition, nutritional properties, and biological activities of Moroccan Spirulina microalga. J Food Qual. https://doi.org/10.1155/2019/3707219
Singleton VL, Rossi JA (1985) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Viticulture 16:144–158. https://doi.org/10.5344/ajev.1965.16.3.144
Sivaramakrishnan T, Swain S, Saravanan K, Sankar K, Roy SD, Biswas L (2017) In vitro antioxidant and free radical scavenging activity and chemometric approach to reveal their variability in green macroalgae from south Andaman Coast of India. Turk J Fish Aquat Sci 17:639–648. https://doi.org/10.4194/1303-2712-v17_3_20
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. https://doi.org/10.1263/jbb.101.87
Thompson PA, Harrison PJ (1992) Effects of monospecific algal diets of varying biochemical composition on the growth and survival of Pacific oyster (Crassostrea gigas) larvae. Mar Biol 113:645–654. https://doi.org/10.1007/BF00349708
Tokuşoglu Ö, Üunal MK (2003) Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J Food Sci 68(4):1144–1148. https://doi.org/10.1111/j.1365-2621.2003.tb09615.x
Uma VS, Gnanasekaran D, Lakshmanan U, Dharmar P (2020) Survey and isolation of marine cyanobacteria from eastern coast of India as a biodiesel feedstock. Biocatal Agric Biotechnol 24:101541. https://doi.org/10.1016/j.bcab.2020.101541
Upadhyay AK, Singh NK, Singh R, Rai UN (2016) Amelioration of arsenic toxicity in rice: comparative effect of inoculation of Chlorella vulgaris and Nannochloropsis sp. on growth, biochemical changes and arsenic uptake. Ecotoxicol Environ Saf 124:68–73. https://doi.org/10.1016/j.ecoenv.2015.10.002
Wickham H (2016) The ggplot2 package license GPL: elegant graphics for data analysis. Springer-Verlag, New York. Retrieved from https://ggplot2.tidyverse.org
Widowati I, Zainuri M, Kusumaningrum HP, Hardivillier Y, Leignel V, Bourgougnon N, Mouget JL (2021) Growth of shrimp infected by Vibrio, fed with formulated feed with inclusions of Dunaliellasalina and Tetraselmischuii extracts. Aquacult Aquar Conserv Legis 14(2):981–987
Widowati I, Zainuri M, Kusumaningrum HP, Susilowati R, Hardivillier Y, Leignel V, Bourgougnon N, Mouget JL (2017) Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. In IOP Conf Ser: Earth and Environmental Science IOP Publishing 55(1):012067. https://doi.org/10.1088/1755-1315/55/1/012067
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T (2012) Effect of high frequency of intermittent light on the growth and fatty acid profile of Isochrysis galbana. Aquaculture 338:111–117. https://doi.org/10.1016/j.aquaculture.2012.01.005
Zhukova NV (2004) Changes in the lipid composition of Thalassiosira pseudonana during its life cycle. Russ J Plant Physiol 51:778–783. https://doi.org/10.1023/B:RUPP.0000040759.04882.8c
Zishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging affects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
The authors acknowledge the support given by the Director, ICAR-CIBA, Chennai for the conduct of the study. The authors are also grateful to the Indian Council of Agricultural Research (ICAR) for the research funding.
Funding
This work was funded by the Indian Council of Agricultural Research (ICAR), New Delhi and the Grant Number was FISHCIBASIL201800800136.
Author information
Authors and Affiliations
Contributions
KP Sandeep and K Ambasankar conceptualized and executed the work; KP Sandeep and J Raymond Jani Angel executed isolation and characterization studies; T Sivaramakrishnan and J Syama Dayal helped in the nutrient profiling of isolates; NS Sudheer and Dani Thomas helped in the antimicrobial assay. KP Sandeep and J Raymond Jani Angel wrote and CP Balasubramanian, M Kailasam and K Ambasankar reviewed the manuscript. All authors agreed to the publication of the manuscript.
Corresponding author
Ethics declarations
Ethical statement
As the present study does not deal with live animals, the ethical statement is not required.
Competing interests
The authors declare there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sandeep, K.P., Sivaramakrishnan, T., Raymond, J.A.J. et al. Nutraceutical potential of microalgae: a case study from a tropical estuary in Southern India. J Coast Conserv 28, 45 (2024). https://doi.org/10.1007/s11852-024-01046-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11852-024-01046-1